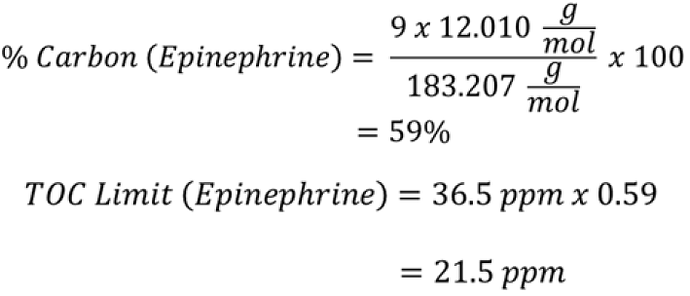Cleaning validation swab sample
PDA Technical Report No. 29: Cleaning validation:
8.0 Maintenance of Validated State
A key part of the validation lifecycle for any system is maintenance of the validated state. A variety of terms are used within the industry for those activities that follow the cleaning process design/development and successful execution of the formal validation protocols. The term used in this Technical Report for those activities is “validation maintenance”; other related terms used in the industry include “continued process verification”, “ongoing process maintenance”, “ongoing process control”, “monitoring”, and “continued process control”. Validation maintenance is critical for cleaning validation because a lapse, shift, and/or change in the validated state has the potential to adversely impact the quality, safety and purity of subsequent batches of the same or different products. The main tools for ensuring the continued maintenance of the validated state are change control, periodic monitoring and data trending review. Additionally, training is an important area of control for cleaning processes, and it is one of the primary mechanisms for controlling manual cleaning consistency.
“7.4.3 Total Organic Carbon”
The rationale for use of TOC in such situations is ease of analytical method development and the worst-case assumptions inherent in TOC analysis.
TOC may be used for all stages of cleaning validation, including design/development, qualification and validation maintenance as well as for investigations.
Suez Application note calculating maximum allowable carryover (MAC) for cleaning validation:
As a nonspecific method, Total Organic Carbon (TOC) analysis measures both product and process related residues as a function of their carbon containing properties. TOC analysis provides efficient feedback necessary to continuously evaluate (verify) the validated state of the cleaning process and, therefore, is compliant with FDA best practice guidance.
Converting product limit to TOC limit
Specific product limits are not directly transferrable to a TOC method. The specific product limit, depending on the sampling method, can be converted to a TOC limit by multiplying by relative mass percentage of carbon from the chemical formula of the product. When a specific method limit has been previously calculated, converting from the specific cleaning validation method, such as HPLC, to a nonspecific method, such as TOC, can be achieved using the percentage carbon in the chemical formula. For example, if a specific API limit for HPLC is established at 10 ppm and the percentage carbon is 50%, the TOC limit would be 5 ppm.
example
With the product limit established (e.g. 36.5 ppm), the TOC limit can be determined by the percent carbon in the chemical formula for Epinephrine, C9H13NO3.
EU (PIC/S) Annex 15: Qualification and Validation:
10.15. Where manual cleaning of equipment is performed, it is especially important that the effectiveness of the manual process should be confirmed at a justified frequency.
10.6.2.If it is not feasible to test for specific product residues, other representative parameters may be selected, e.g. total organic carbon (TOC) and conductivity.
PIC/S : GMP Guide Part I Basic Requirements for Medicinal Products:
iii. Cleaning verification after each product campaign should be considered as a detectability tool to support effectiveness of the Quality Risk Management approach for products deemed to present higher risk;
EMA Q&A on Guideline on setting health based exposure limits:
Q7. Is analytical testing required at product changeover, on equipment in shared facilities, following completion of cleaning validation?
A: Analytical testing is expected at each changeover unless justified otherwise via a robust, documented Quality Risk Management (QRM) process. The QRM process should consider, at a minimum, each of the following:
- the repeatability of the cleaning process (manual cleaning is generally less repeatable than automated cleaning);
- the hazard posed by the product;
- whether visual inspection can be relied upon to determine the cleanliness of the equipment at the residue limit justified by the HBEL.
MHRA’s Q&A document entitled “Cross-contamination control and Health Based Exposure Limits (HBEL)”:
Inspectors are seeing an increasing number of companies who complete cleaning validation and then inappropriately stop conducting sampling and analytical testing for subsequent product changeovers. In drawing a comparison to Process Validation, it would not be acceptable to stop finished product testing just because a process is validated (and this typically does not occur). As such, it is expected that there is analytical assessment at product changeover, supported by method validation and demonstrating adequate recovery.
The only exception to this, as mentioned in Q&A7, is where following successful completion of Cleaning Validation (accounting for all process variables) visual inspection can be relied upon to determine the cleanliness of the equipment at the residue limit justified by the HBEL. This means that there has to be a safety factor applied between what level can be seen on the equipment and the level required to ensure the HBEL is not exceeded – thus a margin of safety is present. In order to meet this, a robust science-based visual threshold study would be required to show what can be seen at specific concentrations on representative surfaces. The safety factor is required to account for the relative ease of seeing contamination in a laboratory setting, in comparison to production setting with variable lighting, access conditions and people, etc involved.
Thankyou very much sir for your response, it is very helpful for me. Some of i got evidence.
I read this PDA 29 where i found that it is non specific method and can be used only for water soluble product.
Can we used it for 100% testing procedure or for only monitoring purpose.
For periodic cleaning verification as illustrated above if needed to be an alternative to the specific analytical procedure which has been used in cleaning validation
Thankyou very much sir.
Acceptance criteria for TOC limit which is mentioned in which guidelines?
I have already mentioned this in the quoted reply
For detailed information which you need I will provide you now with the link of the free full source paper (Suez Application note calculating maximum allowable carryover (MAC) for cleaning validation)
Thankyou very much sir, it’is very helpful for me, again thankyou so much.
Actually I was not confident about TOC analyzer but now I am confident .
This topic was automatically closed 10 days after the last reply. New replies are no longer allowed.