304 VS. 316 Stainless
THE DIFFERENCES BETWEEN TYPE 304 & TYPE 316 STAINLESS STEEL
TYPE 304 STAINLESS STEEL
Type 304, with its chromium-nickel content and low carbon, is the most versatile and widely used of the austenitic stainless steels. Type 304 alloys are all modifications of the 18% chromium, 8% nickel austenitic alloy. Applications for this group of alloys are varied and all possess somewhat similar characteristics in resistance to oxidation, corrosion, and durability. All provide ease of fabrication and cleaning, prevention of product contamination and over a variety of finishes and appearances.
GENERAL PROPERTIES – TYPE 304
Type 304 stainless steels can meet a wide variety of physical requirements, making them excellent materials for applications including auto molding and trim, wheel covers, kitchen equipment, hose clamps, springs, truck bodies, exhaust manifolds, stainless atware, storage tanks, pressure vessels and piping.
TYPICAL ANALYSIS – TYPE 304
Represented by ASTM-A240 AND ASME SA240.
Elements by Percentage by Weight – Maximum Unless Range is Specified.
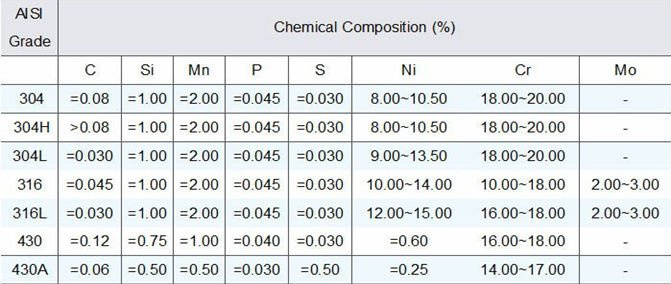
C = .08 / Mn = 2.00 / P = .045 / S = .030 / Si = 1.00 / Cr = 18.00 – 20.00 / Ni = 8.00-12.00 / N = .10
RESISTANCE TO CORROSION – TYPE 304
The 18% chromium, 8% nickel, provides good resistance to moderately acidic or caustic solutions. Type 304 may be considered to perform similarly in most non-severe applications. A notable exception is in the case of welding. Low carbon (304L) is the recommended alloy and provides increased resistance to intergranular corrosion.
MECHANICAL PROPERTIES OF TYPE 304 AT ROOM TEMPERATURE
Typical Mechanical Properties required for annealed material covered by ASTM A240.
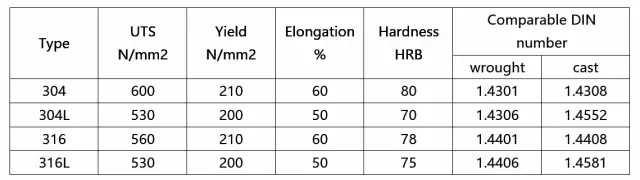
Yield Strength .2% offset = 30,000 / Ultimate Tensile Strength = 80,000 / Elongation = 50%. Hardness R = 90 max.
TYPE 316 STAINLESS STEEL
Type 316 is an austenitic chromium-nickel stainless and heat-resisting steel with superior corrosion resistance as compared to other chromium-nickel steels when exposed to many types of chemical corrodents such as sea water, brine solutions, and the like.
GENERAL PROPERTIES – TYPE 316
Type 316 alloy is a molybdenum bearing stainless steel. It has a greater resistance to chemical attack than the 304 family. Similarly, Type 316 is durable, easy-to-fabricate, clean, weld and finish.
TYPICAL ANALYSIS – TYPE 316
Represented by ASTM-A240 and ASME SA240.
C = .08 / Mn = 2.00 / P = .04 / S = .03 / Si = 1.00 / Cr = 16.00 – 18.00 / Ni = 10.00 – 14.00 / Mo = 2.00 – 3.00
RESISTANCE TO CORROSION – TYPE 316
The addition of 2% molybdenum makes 316 considerably more resistant to corrosion and oxidation than the 304 family of alloys.
MECHANICAL PROPERTIES OF TYPE 316 AT ROOM TEMPERATURE
Typical Mechanical Properties required for annealed material covered by ASTM-A240. Yield Strength .2% offset = 30,000 / Ultimate Tensile Strength = 80,000 / Elongation = 50%. Hardness R = 90 max.
Type 316 is considerably more resistant to solutions of sulfuric acid, chlorides, bromides, iodides and fatty acids at high temperature. In the manufacture of certain pharmaceuticals, stainless steels containing molybdenum are required in order to avoid excessive metallic contamination.
STAINLESS RUST
The basic resistance of stainless steel occurs because of its ability to form a protective coating on the metal surface. This coating is a “passive” film which resists further “oxidation” or rusting. The formation of this film is instantaneous in an oxidizing atmosphere such as air, water, or other fluids that contain oxygen. Once the layer has formed, we say that the metal has become “passivated” and the oxidation or “rusting” rate will slow down to less than 0.002″ per year (0,05 mm. per year).
Stainless gets its non corrosive properties from the chromium in the alloy. It’s created when oxygen combines with the chrome in the stainless to form chrome oxide which is more commonly called “ceramic”. This protective oxide or ceramic coating is common to most corrosion resistant materials. The chromium atoms combines with oxygen and forms a passive surface film over the base steel very much like the paint protects your car. Once this layer is removed the base metal is exposed to the moisture in the atmosphere and rust forms. Chlorine in any form combines with the chromium and removes this protective layer and exposes the base metal and rust will occur.
NEVER use any chemicals that contain chlorine near any stainless. This includes any cleaners, acids to clean quarry tile or brick, and some detergents. Even the vapors can attack stainless steel. The only inorganic acid that is friendly to stainless is nitric and it is used to remove any iron particles left on the surface from manufacturing or machining. Never use steel wool to clean stainless. Particles of the steel wool will get trapped in the grain of the stainless and these steel particles will rust. Halogen salts, especially chlorides easily penetrate this passive film and will allow corrosive attack to occur. The halogens are easy to recognize because they end in the letters “ine”. Listed in order of their activity they are:
- fluorine
- chlorine
- bromine
- iodine
- astatine (very unstable.)
This type of corrosion occurs when there is an overall breakdown of the passive film formed on the stainless steel. It’s the easiest to recognize as the entire surface of the metal shows a uniform “sponge like” appearance. The rate of attack is affected by the fluid concentration, temperature, fluid velocity and stress in the metal parts subject to attack. As a general rule the rate of attack will double with an eighteen degree Fahrenheit rise in temperature (10° C.) of either the product or the metal part.
Chlorides are problematic with austenitic stainless steels like 304 as they can cause pitting, crevice corrosion, and stress corrosion cracking. Temperatures above ambient and cycling between hot and cold temperatures can make corrosion worse as concentration of chlorides due to evaporation can occur. The generally recommended maximum chloride level for 304 stainless steel is only 200 ppm (1000 ppm for 316 stainless steel). The low free chlorine levels of typical potable water systems will not affect austenitic stainless steels. However, free chlorine concentrations of as little as 25 ppm can have a detrimental effect on them. The subject of chloride induced corrosion of austenitic stainless steels is very complex and depends on many things such as concentration, temperature, pH, etc.
A clear epoxy power coating is a tough, durable clear protective coating that protects stainless steel from corrosion, salt air pitting and provides excellent fingerprint and smudge prevention. Stainless steel surfaces protected with a clear epoxy powder finish will be much easier to keep clean and will never darken as it will with oily protectants.