If we change the supplier of excipient used for the manufacture of a pharmaceutical product, is it necessary to revalidate the manufacturing process and carry out a stability study?
Ref. these guidelines
US FDA
- FDA 21 CFR 211 (cGMP for Finished Pharmaceuticals)
→ §211.100 & §211.160: Any change in components (including excipients) must be scientifically justified and validated.
ICH Guidelines
- ICH Q8 (R2) – Pharmaceutical Development
→ Emphasizes understanding material attributes (including excipients) and their impact on critical quality attributes (CQAs). - ICH Q9 – Quality Risk Management
→ Requires risk assessment for changes such as new excipient suppliers. - ICH Q10 – Pharmaceutical Quality System
→ States that supplier changes must go through a formal change control and evaluation process.
WHO Guidelines
- WHO Technical Report Series (TRS 986, Annex 2 – GMP)
→ Notes that changes in raw material suppliers, including excipients, may require process validation or revalidation.
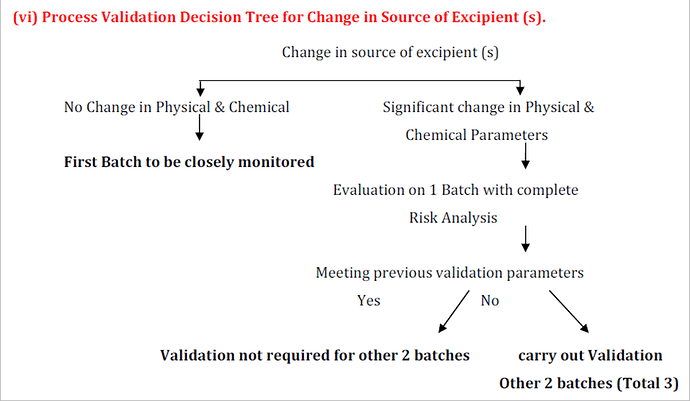
When there is no change in the physicochemical properties of materials from both existing and proposed vendors, re-validation requirements may be reduced. Typically, you can:
- Perform a risk assessment to document that the change does not impact product quality.
- Compare the specifications, manufacturing processes, and impurity profiles of both vendors to ensure equivalency.
- If equivalent, the new vendor may be accepted based on the risk assessment without full re-validation.
Nonetheless, the industry best practice and regulatory expectation is to proceed with re-validation to confirm that processes and systems remain in a validated state over time, minimizing risk and meeting GMP requirements.
Yes — a change in excipient supplier is typically considered a material change under GMP and ICH guidelines. Even if the excipient has the same pharmacopeial grade, differences in particle size, moisture, impurities, or processing aids can affect the product’s critical quality attributes.
![]() What’s usually expected:
What’s usually expected:
- Risk assessment / comparability study of the new vs. old excipient.
- Process revalidation (partial or full) if the risk assessment indicates potential impact.
Stability study (often at least on accelerated and ongoing batches) to confirm no effect on product shelf life.
Regulators (FDA, EMA, etc.) generally require documented evidence that the change does not affect product quality, safety, or efficacy.