- When to perform partical count?
2)If partical counting exeed its limit in various classes how to control ?
3)Why is it important?
1)Usually it is done once a month in sterile filling area.But depends on protocols of Product. Partical counting can be done before start of batch to confirm suitable enviroment for product filling.
- Thorough cleaning of machines and area with disinfectants is required, and area is left for sometime for settle down of particals.For control of particals we must also restrict unnecessarly personal movements and to remove any barriers which disturb the uni directional flow of filtered air.
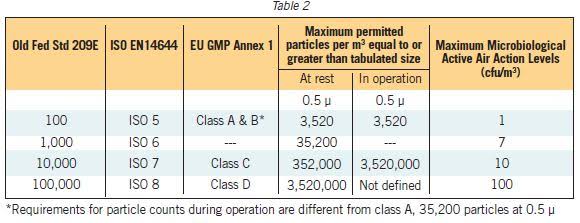
3)It is a practice which verify suitable environment for carring out filling process because particals are the carriers of bacteria from one place to other and Class A is required to be free of any partical.
class A can not be made free from any particles.it contain some particle having range.
0.5 micron r 3520 and 5 micron are 29.
According to European union classification in rest state Partical of 5 micron should be none,These are non viable particles . Usually Partical counter is unable to differentiate between 0.2 and 5 microns it gives only counting of particles . So we should have an aproach towards none,and practically it is possible.
larticle counter differentiate between particles of different sizes.
it gives us different readings for 0.5 and 5 micron particles.
As per revised ISO 14644 Guidelines testing only 0.5 micron. 5 micron particle count test not required.
for sterile area only