Hello, can anyone share a sample dossier for application of sodium chloride 0.9% infusion registration
Please refer to ICH M7 Guidelines on “Common Technical Document”.
ORGANISATION OF THE COMMON TECHNICAL DOCUMENT FOR THE
REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE -M4
Current Step 4 version dated June 15, 2016
The agreement to assemble all the Quality, Safety and Efficacy information in a common format (called CTD - Common Technical Document ) has revolutionized the regulatory review processes, led to harmonized electronic submission that, in turn, enabled the implementation of good review practices. For industries, it has eliminated the need to reformat the information for submission to the different ICH regulatory authorities.
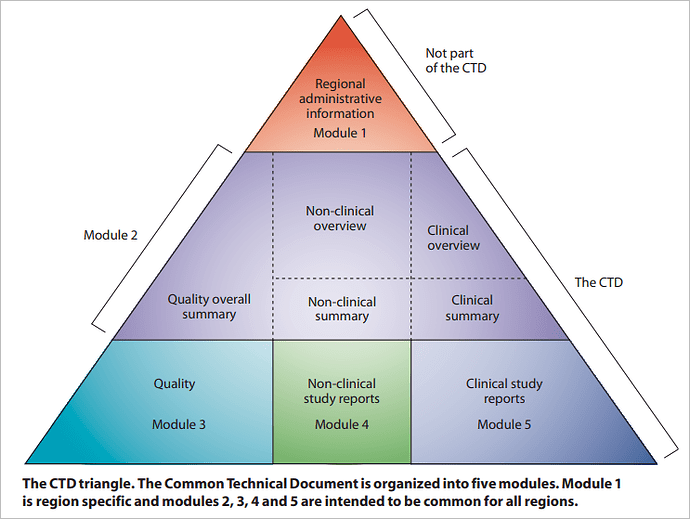
The CTD is organized into five modules. Module 1 is region specific and Modules 2, 3, 4, and 5 are intended to be common for all regions. In July 2003, the CTD became the mandatory format for new drug applications in the EU and Japan, and the strongly recommended format of choice for NDAs submitted to the USFDA, United States.
Thank you
You are always welcome.
This topic was automatically closed 10 days after the last reply. New replies are no longer allowed.