Dear Sir
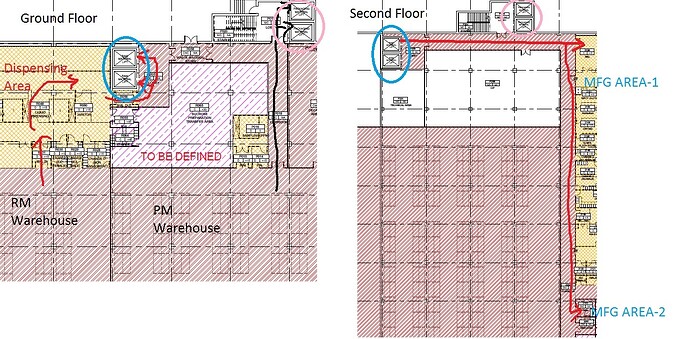
For a vertically designed multi-dosage liquid/semi-solid plant (oral liquid, suppository, cream ointment, softgel etc), it is considered that central dispensing booth (separate API & Excipients booth) is attached to RM warehouse at Ground floor. All manufacturing area is considered at Second floor with segregated zone/compartment by CNC corridor passage entrance with dedicated change facilities.
Clean class of dispensing area is designed as Class D and manufacturing area are either C or class D. The dispensed materials will be transferred by goods lift to second floor and then it will travel through a CNC corridor to various MFG area by a material air lock or dynamic pass box (depending on pack size).
So after dispensed, properly seal bags of RM are travel by closed movable trolley and pallet as following sequence to deliver the RM for MFG area.
Dispensing Class D (pallet transfer) → Air Lock (pallet → closed trolley) → CNC lobby (closed trolley) → Goods lift (CNC) → CNC corridor (closed trolley) → Air lock (pallet) → Class D (pallet) → Air Lock (pallet → pallet) → Class C.
In various guidelines, dispensing and weighing section gives emphasis on clean equipment and clean environment but not mention any transfer path criteria of dispensed material.
My questions is, the above designed material transfer system (liquid/semi-solid plant) is acceptable to GMP/QbD point of view? Need your kind suggestion.