Dear All ,
There was a question in my mind related to Deviation and Lab incident.
The question is related to GC or HPLC analysis. Suppose during the sample analysis bracketing standard injection not acquired and sequence got interrupted due to any reason. If repeated analysis will be there, then it is an Incident or will be addressed through Deviation. Please clear.
Since it is a unplanned event it shall be handled through QC incident.
Hi Sir,
But I observed that in south India region mostly companies are logging deviation and making investigation report through the deviation procedure.
As per their concept, if anything will be happened before the sample then that will be incident and after sample injection will be a deviation.
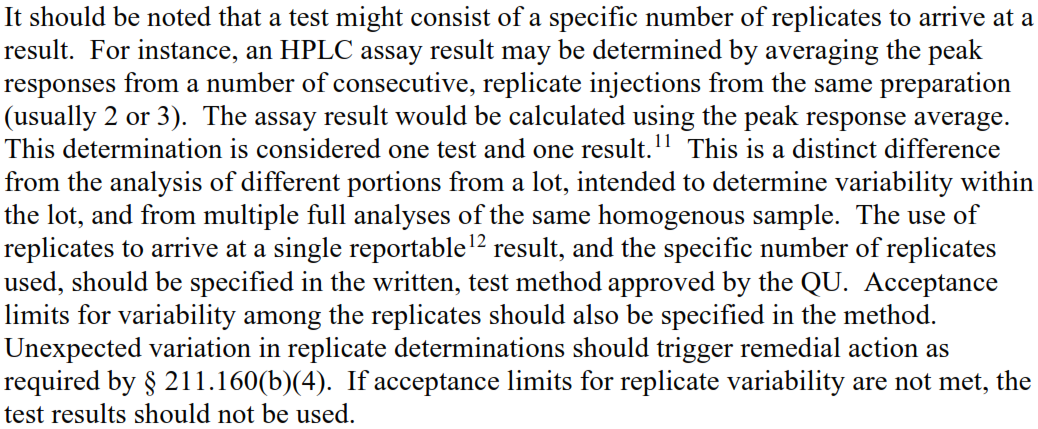
The following is quoted from FDA guidance on OOS investigation
https://www.fda.gov/media/158416/download
Hi Dr. Ahmed Mohamed Awad,
My question was related to Lab Incident and Deviation, not related to OOS. Thanks for your reply.
1 Like
This topic was automatically closed 3 days after the last reply. New replies are no longer allowed.