How many change rooms and air locks are required for designing Microbial limit testing area and Bacterial endotoxin area.
1 Like
- From Non grade to D and D to C for this test.
I think it’s 4. But need to confirm.
Based on WHO guideline it doesn’t need to be taken in a Classified area, Just taking under a LAF is enough.
Please what is the name or the link of this WHO guideline
WHO Technical Report Series, No. 961, 2011; Annex 2 WHO good practices for pharmaceutical microbiology laboratories
1 Like
You Mean unclassified room and LAF is enough.?
What about background for LAF?
What about change room?
Really Thanks a lot
It depends on the output of your risk assessment analysis or your local GMP rules. In my country, LAF for MLT shall be located in Grade D but it is not mandatory for example in EU it most countries CNC+ might be enough.
1 Like
Does anyone have example micro lab layouts for microbial limit testing?
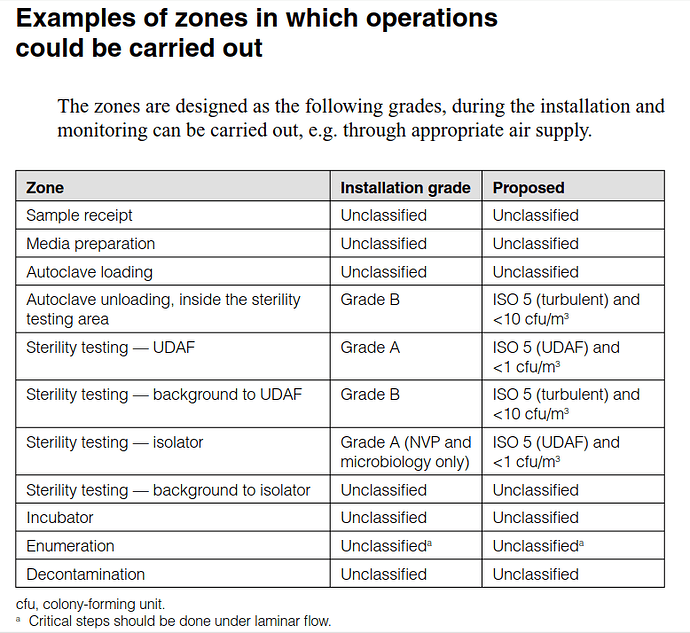
Check it